The particle of matter
Properties |
Solids |
Liquids |
Gases |
| Molecular Structure |
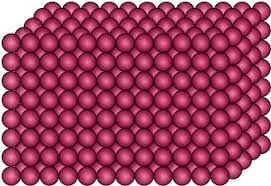 |
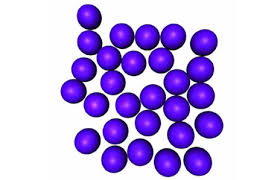 |
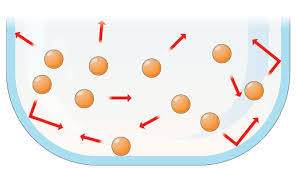 |
volume |
Fixed volume |
Fixed volume |
No Fixed volume |
Shape |
Fixed shape |
No Fixed Shape :takes the shape of the container |
No Fixed Shape |
When Pressure is Applied |
Incompressible |
Incompressible |
Compressible |
Collisions |
Do Not Collide |
Collide Often |
Not Many Collisions Happen |
| Movement of particles |
Particles Vibrate about Fixed Positions |
Some are Free to Move |
Particles are Free to Move and Move in Random Directions |
Kinetic Theory Of Matter
- All matter is made of tiny moving particles
- Different substances have different types of particles; atoms, molecules and ions, which all have different sizes
- These particles are always moving
- The higher the temperature the faster they move on average
- The smaller the particles are the faster they move
